Research on Radiation Injury
Hyperbaric oxygen in the treatment of delayed radiation injuries of the extremities
By J.J. FELDMEIER, R. D. HEIMBACH, D. A. DAVOLT, M. J. MCDONOUGH, B. J. STEGMANO, and P.J.SHEFFIELD
Medical College of Ohio. Toledo. Ohio; and The Jefferson C. Davis Wound Care and Hyperbaric Center. San Antonio. Texas
Feldmeier JJ, Heimbach RD, Davolt DA1 McDonough MJ, Stegmano BJ, Sheffield PJ.
Hyperbaric oxygen in the treatment of delayed radiation injuries of the extremities. Undersea Hyper Med 2000; 27(1)15-19.-Hyperbaric oxygen (HBO2) is used as an adjunct in the treatment of radiation injury at many sites, including the mandible, larynx, chest wall, bladder1 and rectum. In these disorders, HB02 is effective in stimulating neovascularization and reducing fibrosis. No previous publications report the application of HB02 to radiation injuries of the extremities. From 1979 until 1997, 17 patients were treated at the Southwest Texas Methodist and Nix Hospitals for nonhealing necrotic wounds of the extremities within previously irradiated fields. All but one wound involved a lower extremity. Most of the patients had been irradiated for soft tissue sarcomas or skin cancers. The rest were irradiated for a variety of malignancies. HB02 was delivered in a multiplace chamber at 2.4 atm abs daily for 90 min of 100% oxygen at pressure. This report is a retrospective, uncontrolled review of these patients. Eleven patients (65%) healed completely whereas five (29%) failed to heal and one (6%) was lost to follow-up. Three (60%) of those who failed were found to have local or distant recurrence of their tumor early in their course of hyperbaric treatment and were discontinued from therapy at that time. When last seen in the clinic, the wound of the patient who was lost to follow-up was improved but not completely healed. Four of those who failed (including the two with local tumor recurrence) required amputation. If we exclude those with active cancer and the patient lost to follow-up, the success rate was 11 of 13 or 85%. HB02 was applied successfully with complete wound healing and the avoidance of amputation in a majority of these patients. The consequences of failure in patients suffering from radiation necrosis of the extremities (some complicated by the presence of tumor) are significant, with 80% of the five failures requiring amputation. In radiation injuries of the extremities as in delayed radiation injury at other sites, HBO2 is a useful adjunct and should be part of the overall management.
One of the most successful and best documented indications for hyperbaric oxygen (HB02) has been its application to the treatment and prevention of mandibular osteoradionecrosis (1-3). Hyperbaric oxygen for the treatment of soft tissue and bony necrosis at other sites is an indication approved by the Hyperbaric Oxygen Therapy Committee of the Undersea and Hyperbaric Medical Society, and reimbursement is approved for this indication by Health Care Finance Administration and other third-party payers. A number of papers report success in applying HB02 to radiation injuries at anatomic sites other than the mandible, including chest wall, abdomen, larynx, small bowel, large bowel, and bladder (419). However, a review of the literature fails to discover any reports of HB02 applied to delayed radiation injuries of the extremities. The postulated mechanism for HBO2 in the treatment of radiation necrosis of the extremities is the induction of angiogenesis and the reduction of fibrosis. In turn, these changes provide the oxygen necessary to support healing and resolution of necrosis. Marx and colleagues (20,21) have shown an increased density of vasculature after HBO2 in both clinical histologic specimens and in an animal study with microangiography. Feldmeier and associates (22,23) have demonstrated a reduction of fibrosis in an animal model of enteritis when HB02 is delivered to animals who have received radiation exposures likely to cause significant delayed complications.
MATERIALS AND METHODS
Since 1979, we have treated nine women and eight men with HB02 for radiation necrosis of the extremities. Sixteen of these 17 patients are available for follow-up. Malignancies irradiated included several different types and are listed in Table 1. The patient's ages ranged from 21 to 87 with a median age of 64. The interval from radiation therapy to injury varied from 4 wk to 17 yr, with a median time of 21 mo. The time from the manifestation of the injury to the referral for HB02 varied from immediately to 3 yr, with a median time of 6 mo. None of the patients had had any progression to healing in that time interval using a variety of conservative therapies including wound care and antibiotics. Two of 17 patients were referred less than 2 yr after their irradiation. Radiation doses varied from 3,600 to 6,000 cGy with a median dose of 6,000 cGy. Five of 17 patients had doses less than 6,000 cGy. The vast majority of injuries (17 of 18) involved the lower extremity. One patient had radiation necrosis of the upper extremity. Seventeen of the 18 wounds demonstrated only soft tissue involvement while the one patient with upper extremity involvement had bony as well as soft tissue involvement. Patient number 16 was treated for an original ulcer over the anterior tibia, with resolution after 43 treatments. About 9 mo. after this first course of therapy, the patient had a recurrence of the wound, although smaller. This recurrent wound was treated with a second course of HB02. Table 2 gives a complete description of pretreatment characteristics.
Table 1: Pre-Treatment Characteristics
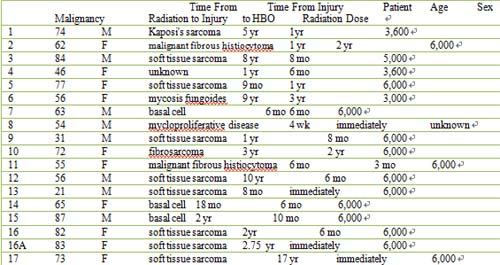
Key: STSG split thickness skin graft; aDose approximated from standard radiation therapy guidelines.
The HB02 exposures consisted of 90 min of 100% oxygen at 2.4 atm abs in a multiplace hyperbaric chamber. Treatments were typically given in three periods of 30 min 02 breathing with l0-min air breaks interposed between 02 periods. A medical attendant was always present in the chamber during the treatment. Daily wound care was an important part of the patients' therapeutic regimen. Typically, wounds received high-pressure irrigation with normal strength saline solution. Sharp debridement was done as necessary and as tolerated to remove superficial necrotic tissue. Wounds were then usually dressed with a "wet-to-dry" dressing using dilute boric acid.
Surgical intervention was used as appropriate in each case. Nine of the 17 patients underwent at least one surgical procedure as part of the therapy designed to preserve their limb. The specific surgical interventions are listed in Table 2.
RESULTS
Sixteen patients (94%) with 17 wounds were available for analysis. The full details of the patients' outcome are included in Table 2. One patient was lost to follow-up. This patient had shown significant improvement at 43 treatments. A flap was planned for this patient and complete resolution was expected but not documented. Eleven of the remaining 16 patients had complete healing of their necrotic wounds. The successfully treated patients were all followed to healing which was completed by the final HB02 treatment or within a week or two after treatment. As noted above, patient number 16 had initial resolution of her wound, but it recurred and required a second course of HB02 for permanent resolution. The successfully treated patients included the one with bony as well as soft tissue involvement of the upper extremity. In the 11 patients who healed completely, 3 of 11(27%) required no surgery; 3 of 11(27%) had skin grafting; 2 of 11(18 %) had surgical debridement and 1 of these had delayed primary closure. 1 of 11(9%) had had a surgical resection of recurrent tumor just before beginning HB02; 1 of 11(9%) had debridement along with bone and skin grafting; and 1 final patient (9%) had both a myocutaneous flap and split thickness skin graft. (Note: The numbers do not add up to 100% due to rounding.) The timing of surgery relative to the course of hyperbaric treatments is included in Table 1. Since these patients were not treated according to a planned prospective trial, considerable variation in relative timing of surgery to HB02 is seen. Surgery was accomplished when the referring surgeon felt that the timing was optimal for each patient. However with the exception of patient number 12, all patients had at least 20 HBO2 treatments before any surgical intervention. No flap or graft loss was experienced in our group of patients.
Table 2: Wound Characteristics, Treatment, and Outcome
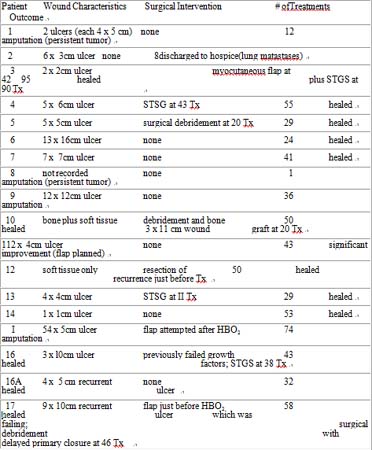
The number of HB02 treatments varied from 1 to 95. For those who healed, the number of treatments varied from 24 to 95 with a median number of 47. For those who failed to heal, the number of treatments varied from 1 to 74 with a median number of 24. Patients' treatments were continued until clinical judgment determined that maximum benefit had been obtained or until biopsy of the lesion demonstrated tumor presence in the wound or, in one case, lung metastases was detected.
Of the five (29%) who failed to heal, four underwent amputation. Two (12%) of these (patients numbers 1 and 8) were found to have persistent or residual tumor in their wounds and were discontinued from hyperbaric treatment as soon as recurrence was demonstrated after 12 and 1 treatments, respectively. Another patient (6%) in this group was found to have lung metastases after eight HB02 treatments and was placed in hospice care at that time. If we exclude those with local or metastatic malignancy, only two patients failed. Including only these failures and excluding the patient lost to follow-up, the success rate was 85%(1l of 13).
The results of treatment are exemplified by patient number 10 whose serial wound photographs are depicted in Fig. 1. This case was selected for several reasons: her injury involved damage to both soft tissue and bone, and therefore successful intervention was a more significant accomplishment than closure of a soft-tissue only wound. Furthermore a long-term follow-up photograph taken 14 yr after successful intervention was available and confirmed that the effects of HB02 in the treatment of radiation damage of the extremities provides a durable repair of the injury.
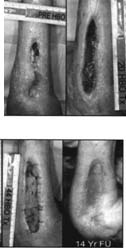
FIG. 1 -Patient 10. Top left: at the time of initial consultation for HBO2; top right: after 30 HB02 treatments status post debridement and bone graft at 20 treatments; bottom left: after 44 HBO2 treatments status post-STSG at 40 treatments; bottom right 14 yr after completion of HBO2 with a functional and cosmetically reasonable outcome.
DISCUSSION
The above report is a retrospective review of all patients treated by the Methodist-Nix Hospital group in San Antonio since 1979 for a diagnosis of radiation necrosis of the extremities. A search of the literature failed to discover specific reviews of radiation injury of the extremities. Several publications do discuss radiation injuries of the skin and subcutaneous soft tissues in general. Necrosis of the skin as a late complication of irradiation has been reviewed recently by Emami et al. (24). In this review for a 10 x 10 cm field, a 5% incidence of necrosis occurred at a dose of 5,500cGy. Fajardo (25) indicates that late skin ulceration occurs in 5% of patients at a dose of 6,000 cGy and 50% at a dose of 8,000 cGy. In our report most patients had been irradiated for skin cancer or soft tissue sarcomas and only 5 of 17 had doses of radiation less than 6,000 cGy. Two patients who had radiation exposures in the lower dose range had ulcerated wounds due to persistent tumor rather than to radiation injury. Radiation damage to bone and skin overall is less likely with today's techniques than it had been in the pre-cobalt days of radiation therapy. (25,26). This decrease is due to the physical characteristic of modern radiation modalities which provide a lower dose in the skin relative to deep seated tumors and avoid selective deposition of the radiation dose in bone. With an increased emphasis on limb preservation in soft tissue sarcomas of the extremities, we may actually see an increase in necrosis of the extremities in the future (27). Gurlek et al. (27) have reported their results in using free tissue transfers to treat a variety of delayed radiation injuries of the head and neck, chest wall, groin, and extremity. A total of 33 patients are reviewed in this paper which 4 had injuries of the extremities. HB02 was not use in this series. Thirty-three flaps were accomplished in this group with an eventual success rate of 97%. However. three flaps were endangered by thrombosis and one was lost. A total of 40% of patients suffered complications including flap loss, partial flap loss, dehiscence, cellulitis, and seroma.
Our series also suggests that the lower extremity is higher risk than the upper extremity for delayed radiation injury, although this relative increase may be due to higher incidence of tumors of the lower extremity, which undergo irradiation as part of their management (28). Our experience with diabetes suggests that the lower extremity is more likely to develop non-healing wounds when a pathology (either radiation injury or diabetes) that induces small vessel damage is superimposed on the inherent relatively diminished blood supply of the lower extremity.
In those patients treated for radiation injury of the extremities who were cancer free, resolution of necrosis with healing of the necrotic wound occurred 85% of the time with avoidance of amputation and maintenance of a useful limb. In the three patients with demonstrated active malignancy it is impossible to say whether an intercurrent depression of their immune response contributed to their wounds and failure to heal. None of the patients were undergoing chemotherapy or radiation therapy at the time of their HB02 treatments. All were also deemed to have adequate nutritional status to support wound healing. All three of these patients had 12 or fewer hyperbaric treatments and two the three had biopsy proven tumor in the wound itself Only two patients (numbers 9 and 15) failed without evidence of recurrent tumor. Both of these had sizable ulcers before HB02 and both had doses of 6.000 cGy. Theyboth failed in spite of adequate trials of hyperbaric treatment. Their failures indicate that though effective in most cases HB02 is not universally successful in the treatment of radiation injuries. HB02 is recommended as part of the management of delayed radiation injuries of the extremities. Care must be taken to rule out recurrent or persistent tumor as an etiology of apparent necrosis and non-heating wounds. Our review also suggests that preoperative HB02 decreases the likelihood of surgical complications as compared to at least one publication where HBO2 was not used (27).
REFERENCES
1. Mainous EG Hyperbaric oxygen in maxillofacial osteomyelitis, osteoradionecrosis and osteogenesis enhancement. In: Davis JD, Hunt TK, eds. Hyperbaric oxygen therapy. Bethesda, MD:Undersea Medical Society, 1977:191-203.
2. Marx RE. Osteoradionecrosis of the jaws: review and update. HBO Rev 1984: 5:78-126.
3. Marx RE, Ames JR. The use of hyperbaric oxygen in bony reconstruction of the irradiated and tissue-deficient patient. J Oral Maxillofac Surg 1982; 40:412-420.
4. Hart GB. Mainous EG. The treatment of radiation necrosis with hyperbaric oxygen. Cancer 1976; 37:2580-2586.
5. Ferguson BJ, Hudson WR, Farmer JC. Hyperbaric oxygen for laryngeal radiation necrosis. Ann Otol Rhinol Laryngol 1987; 96:l-6.
6. Feldmeier JJ, Heimbach RD, Davolt DA, Brakora MJ. Hyperbaric oxygen as an adjunctive treatment for severe laryngeal necrosis: a report of nine consecutive cases. Undersea Hyper Med 1993; 20:329-335.
7. Feldmeier JJ, Heimbach RD, Davolt DA, Court WS, Stegmann BJ, Sheffield PJ. Hyperbaric oxygen as an adjunctive treatment for delayed radiation injury of the chest wall: a retrospective review of twenty-three cases. Undersea Hyper Med 1995; 22:383-393.
8. Warren DC, Feehan P, Slade JB, Cianci PE. Chronic radiation proctitis treated with hyperbaric oxygen. Undersea Hyper Med1997; 24:181-184.
9. Feldmeier JJ, Heimbach RD, Davolt DA, Court WS, Stegmann BJ, Sheffield PJ. Hyperbaric oxygen as an adjunctive treatment for delayed radiation injuries of the abdomen and pelvis. Undersea Hyper Med 1996; 23:205-213.
10. Weiss JP, Boland FP, Mori H, Gallagher M, Brereton H Preate DL. Treatment of radiation-induced cystitis with hyperbaric oxygen. J Urol 1985; 134:352-354.
11. Schoenrock GJ. Cianci P. Treatment of radiation cystitis with hyperbaric oxygen. Urology 1986; 27:271-272.
12. Weiss JP, Nevill EC. Hyperbaric oxygen: pnimary treatment of radiation-induced hemorrhagic cystitis. J Urol 1989: 142:43-45
13. Rijkmans BG. Bakker DJ, l)abhoiwala NF, Kurth KH. Successful treatment of radiation cystitis with hyerbanc oxygen. Eur Urol 1989; 16:354-356.
14. Norkool DM, Hampson NB, Gibbons RE. Weissman RM. Hyperbaric oxygen for radiation-induced hemorrhagic cystitis. J Urol 1993; 150:332-334.
15. Lee HC, Liu CS, Chiao C, Lin SN. Hyperbaric oxygen therapy in hemorrhagic cystitis: a report of 20 cases. Undersea Hyper Med l994; 21:321-327.
16. Akiyama A, Ohkubo Y, Takashima R, Furugen N, Tochimoto M, Tsuchiya A. Hyperbaric oxygen in the successful treatment of two cases of radiation-induced hemorrhagic cystitis. Jpn J Urol 1994; 85:1269-1272.
17. Weiss JP, Mattei DM. Neville EC, Hanno PM. Primary treatment of radiation-induced hemorrhagic cystitis with hyperbaric oxygen: 10-year experience. J Urol 1994; 151:1514-1517.
18. Bevers RF, Bakker DJ, Kurth KH. Hyperbaric oxygen treatment for haemorrhagic radiation cystitis. Lancet 1995; 346:803-805.
19. Miyazato T, Yusa T, Gnaga T, et al.. Hyperbaric oxygen for radiation-induced hemorrhagic cystitis. Jpn J Urol 1998: 89:552-556.
20. Marx RE, Johnson RP. Problem wounds in oral and maxillo-facial surgery: the role of hyperbaric oxygen. In: Davis JC, Hunt TK. eds. Problem wounds: the role of oxygen. New York: Elsevier, 1988:65-123
21. Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg 1990; 160:519-524
22. Feldmeier JJ, Jelen I, Davolt DA, Valente PT,, Meltz ML, Alecu R. Hyperbaric oxygen as a prophylaxis for radiation induced delayed enteropathy. Radiother Oncol 1995; 35:138-144.
23. Fcldmeier JJ, Davolt DA, Court WS, Onoda JM, Alecu R. Histologic morphometry conf'irms a prophylactic effect for hyperbaric oxygen in the prevention of delayed radiation enteropathy. Undersea Hyper Med 1998; 25:93-97.
24. Emami, B, Lyman 3, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21:109-122.
25. Fajardo LF. Pathology of radiation injury. New York. NY: Masson Publishing, 1982:186-187.
26. Rubin P, Casarrett GW. Cljnical radiation pathology, vol. 1. Philadelphia, PA: WB Saunders, 1968:107-109.
27. Gurlek A, Miller MJ, Amin AA, et al. Reconstruction of complex radiation-induced injuries using free-tissue transfer. J Reconstr Microsurg 1998; 14:337-347
28. Lawrence W, Donegan W, Natarajan N et al. Adult soft tissue sarcomas: a pattern of care survey of the American College of Surgeons. Ann Surg 1987; 205:349-355.
For those interested in receiving HBOT that may have difficulty with financing the treatments, we have people that can give advice on fund-raising. The previous case studies are only a glimpse of the hundreds of people which have received HBOT at the Richmond Hyperbaric Center for a variety of conditions with favorable outcomes. Please contact us for more information on a particular condition which may benefit from hyperbaric oxygen therapy.
The Richmond Hyperbaric Health Center has engaged in scientific research projects including a pilot study on the treatment of RSD/CRPS. We would like to pursue future research studies as well and are open to any inquiries about the use of HBOT for researching various conditions.

